LymphoVista
LymphoVista is a cutting-edge liquid biopsy lymphoma test that enables non-invasive detection and monitoring of lymphomas using circulating tumor DNA (ctDNA).
Vista ctDNA Test Family for Lymphomas
Our Vista ctDNA assays use disease-specific gene panels to deliver precise MRD lymphoma detection and continuous therapy monitoring through blood-based ctDNA analysis.
High Precision
Detects and monitors lymphoma mutations with exceptional sensitivity.
Tumor-Specific Panels
LymphoVista for all B-cell lymphomas, LymphoVista HL for Hodgkin lymphomas - each with optimized gene panels.
Technical Specifications
LymphoVista
B-Cell Lymphomas
Technical Validation
Variant Detection: 93.86% sensitivity and 99.999% specificity for variants with ≥0.5%
mAF
MRD Detection: Limit of detection (LoD): 6.69 × 10⁻⁶; 100% accuracy for MRD values >3.04
× 10⁻⁵1
Clinical Validation
Clinical validation in large B-cell lymphoma with 326 samples from 88 patients: MRD-negative patients showed 77% vs. 33% 18-month OS (HR 4.61, p<0.0001) and 51% vs. 5% 18-month PFS (HR 4.32, p<0.0001)2
Application
Indications: e.g. (D)LBCL, follicular lymphoma, mantle cell lymphoma, marginal zone
lymphoma, Burkitt lymphoma, CNS lymphomas
Gene Spectrum: optimized for B-cell lymphomas
Sample Material: 20 ml blood in cfDNA tubes
Time to Result: 2-4 weeks
LymphoVista HL
Hodgkin Lymphoma
Technical Validation
Variant Detection: 91.27% sensitivity and 99.99% specificity for variants ≥0.5% mAF
MRD Detection: Limit of detection (LoD): 6.54 × 10⁻⁶ with 100% accuracy for MRD values
>1.76 × 10⁻⁵1
Clinical Validation
Clinical validation in advanced Hodgkin lymphoma: MRD-2-positive rate: 18.5%. 4-year PFS: 95.3% in MRD-2-negative vs. 72.2% in MRD-2-positive patients (HR 6.9, p<0.0001). Prognostic in both PET-2-positive and PET-2-negative patients.1
Application
Indications: Hodgkin Lymphoma
Gene Spectrum: Slightly modified panel, specifically optimized for Hodgkin lymphoma
Sample Material: 20 ml blood in cfDNA tubes
Time to Result: 2-4 weeks
Clinical Benefits
MRD Detection
Highly sensitive detection of minimal residual disease, often earlier than imaging methods.
Therapy Monitoring
Continuous ctDNA-based monitoring of therapy response.
Early Relapse Detection
Identify relapses months before they appear radiologically.
Risk Stratification
Accurate relapse risk assessment for personalized treatment decisions.
Our tests complement broader liquid biopsy MRD workflows, allowing integration with other molecular and imaging diagnostics.
Clinical Evidence
Our technology has been validated in multiple clinical studies and presented at major hematology congresses. For details and publications, visit our publications section.
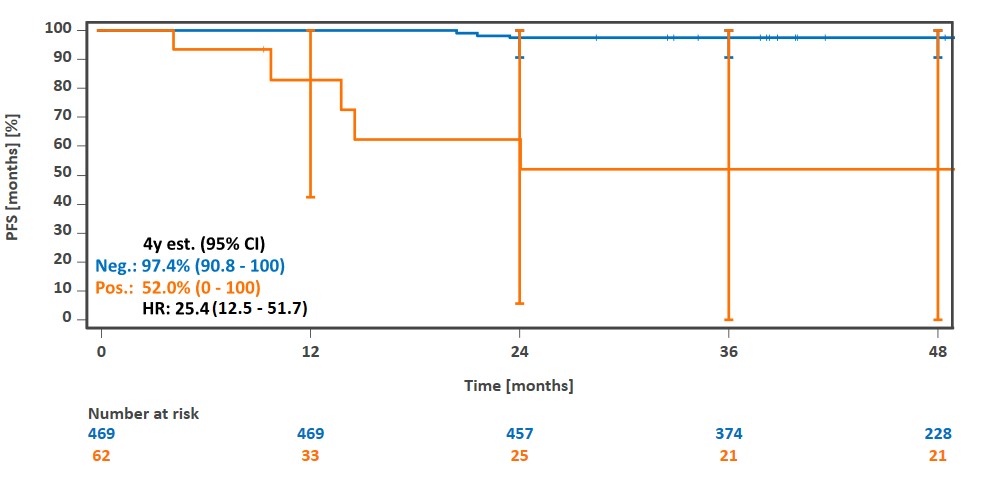
Progression-free survival depending on positive or negative MRD test after 2 cycles of BrECADD chemotherapy in Hodgkin lymphoma1. Details can be found in the references.
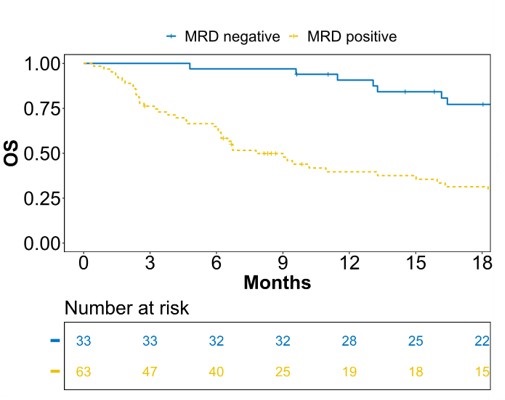
Overall survival depending on positive or negative MRD after relapse treatment of patients with large B-cell lymphoma2. Details can be found in the references.
Test Workflow
Genotyping (Initial Test)
Identification of patient-specific mutations from blood or tissue sample to create an individual mutation profile.
MRD Monitoring
Regular ctDNA testing of known mutations to detect minimal residual disease in follow-up samples.
Report & Interpretation
Detailed report with medical interpretation, available after 2-4 weeks via password-protected email.
References
1 Mattlener et al. Presentation at American Society of Hematology (ASH) Annual Meeting 2024
2 Schleifenbaum et al. Presentation at European Hematology Association (EHA) Annual Meeting 2024
Interested in LymphoVista?
Contact us to discuss your study or clinical application, or to request testing through the LymphoVista platform.